Accelerating Ethiopia’s Production of Critical COVID-19 Supplies
April 27th, 2020 | news
In just two weeks, the USAID Digital Health Activity (DHA) team created systems to support the Ethiopian Food and Drug Administration’s (EFDA) efforts to accelerate the availability of critical personal protective items needed to combat COVID-19. The DHA team applied digital solutions to shorten the amount of time it takes manufacturers to register critical supplies from a minimum of three days to one.
In response to the COVID-19 pandemic, the EFDA is granting manufacturers temporary certificates of competency (COC) to mass-produce hand sanitizers. DHA developed a system to enable EFDA to control hand sanitizer quality as part of the electronic regulatory information system (eRIS) that facilitates registration and import approval for food and medicines.
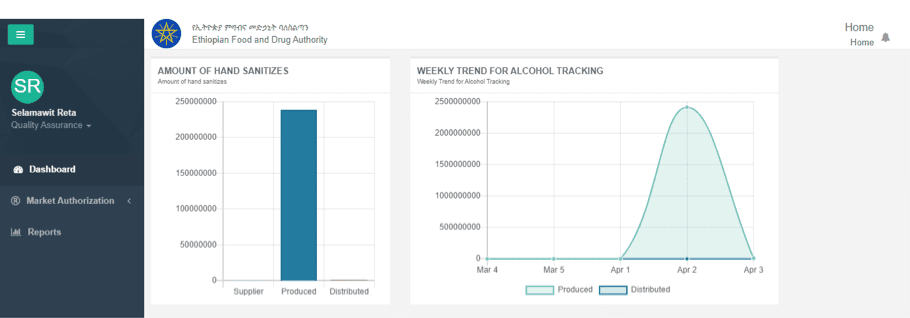
Manufacturers that receive a temporary license to produce alcohol-based sanitizer now use DHA’s quality control system to track the quality of sanitizers they are producing in accordance with World Health Organization standards. Manufactures enter data, including alcohol quantity, source, batch number, quantities of hand sanitizer produced and distributed, into the system on a daily basis. EFDA analyzes the reports to determine hand sanitizer quality and takes action to recall any products that fail quality control checks. As a result, EFDA is able to oversee the multitude of producers to ensure that high-quality products circulate in the marketplace.
A subsystem of the EFDA’s eRIS is iLicense, which issues COCs for manufacturers, importers, wholesalers, and exporters of food and medicine. iLicense allows applicants to apply for and receive product licenses.
COVID-19 preparedness required a simplified version of iLicense to expedite the licensing and registration of items related to the pandemic. The DHA team stepped in to develop a simplified process of issuing temporary COCs to manufacturers.
In this latest version of eRIS, applicants for expedited licensing, such as alcohol-based sanitizer manufacturers, are given priority and do not have to enter the existing queue. The normal licensing process has been reduced to three major activities with fewer documentation requirements:
This version is designed to decrease the amount of physical time applicants spend at the EFDA, protecting both the applicants and EFDA staff from unnecessary physical contact. It also increases transparency and efficiency, allowing goods to reach the market more quickly. Normally, it would take manufacturers three or more days to obtain licenses. Thanks to DHA’s intervention, manufacturers can now get their license the same day they apply for it.

We strive to build lasting relationships to produce better health outcomes for all.