Building a Resilient Medicine Supply Chain to Combat Tuberculosis in the Kyrgyz Republic
March 24th, 2022 | viewpoint
This blog was originally posted on usp.com
Preventing, treating, and curing patients with drug-resistant tuberculosis (DR-TB) is a major public health challenge in the Kyrgyz Republic and worldwide. In fact, the Kyrgyz Republic has one of the highest rates of multidrug-resistant tuberculosis (MDR-TB) in the world.
In 2020, nearly 7,000 people in the Kyrgyz Republic developed TB, 1,000 of whom were diagnosed with DR-TB.
According to the World Health Organization (WHO), 29 percent of new TB cases in the Kyrgyz Republic are DR-TB, compared with 3.3 percent worldwide.
Robust and resilient medicine supply chains capable of consistently delivering quality-assured diagnostics and medicines are critical to the diagnosis and treatment of TB, including MDR-TB. Yet, too often disruptions jeopardize the integrity of supply chains and can directly harm patients, especially vulnerable and hard-to-reach communities.
Disruptions in the medicine supply chain, including stockouts, shortages of quality-assured medicines, and drug substitutions, can cause life-threatening outcomes for patients. Supply chain disruptions force patients into an untenable position of having to resort to sourcing TB medicines by informal means, where quality is not assured and they may be at higher risk for developing DR-TB.
As a first step in building resilient supply chains, it is important to assess existing vulnerabilities, including critical points where quality may be compromised. These can include an over-reliance on a single supplier, opaque and convoluted sourcing and procurement processes, insufficient regulatory oversight, and inadequate quality testing of TB medicines.
Too often, when we think about the medicine supply chain, we think about logistics and procurement. But losing sight of how important assuring quality is across the supply chain can put patients at risk.
Measures that help safeguard quality across the supply chain:
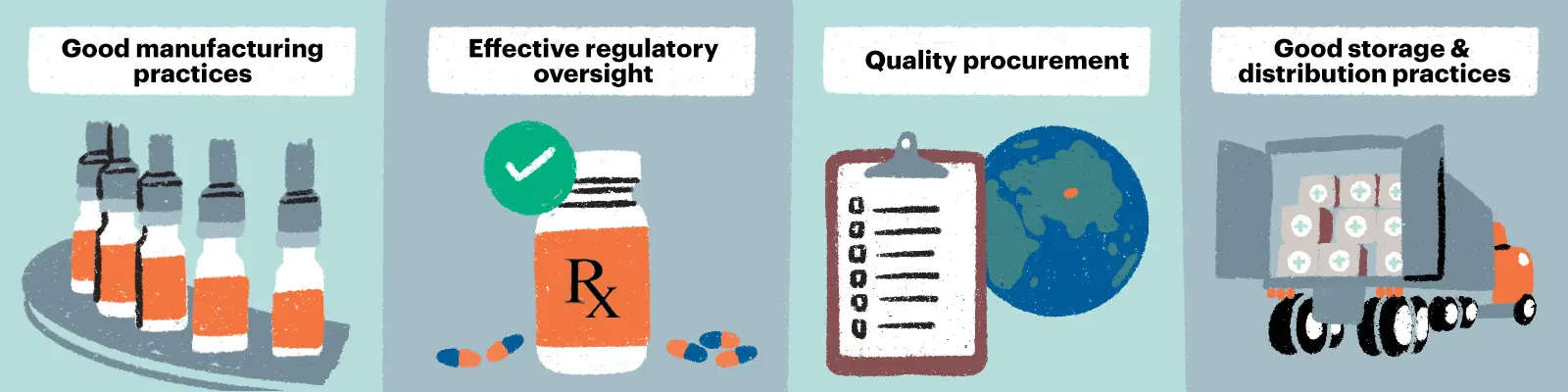
If quality is compromised at any point in the supply chain, TB medicines may not work as intended and could foster the development of drug resistance. For patients with MDR-TB, quality-assured medicines are a lifeline and any interruptions could lead to extensively drug-resistant TB, a particularly dangerous diagnosis with few treatment options.
Poor-quality TB medicines contribute to drug resistance, harming patients, and reducing the effectiveness of medical treatment.
As part of its goal to reduce the TB burden and ensure a consistent supply of quality-assured TB medicines in the Kyrgyz Republic, the U.S. Agency for International Development (USAID) Cure Tuberculosis project, led by JSI Research & Training Institute, Inc (JSI) in partnership with University Research Co., LLC (URC) and USP, is working to improve diagnosis and treatment for people with DR-TB, particularly in hard-to-reach populations. Together, JSI, URC, and USP are providing technical support to improve TB diagnosis by strengthening the national laboratory system and improving access to and management of quality-assured TB medicines.
Specifically, USP is working to assure and safeguard the quality of TB medicines across the supply chain and to expand the supply of these essential medicines in the Kyrgyz Republic. This requires a holistic approach, from assessing medicines manufacturers and regulatory review processes, to evaluating procurement policies and good storage and distribution practices, to improving diagnostic laboratory networks and quality testing at strategic points in the supply chain.
Following an end-to-end assessment of quality assurance across the TB medicine supply chain, we are now working with local counterparts to integrate quality assurance into the National Strategy on TB Control, which will guide the country’s response to TB for the next five years.
At the same time, we are working closely with the Kyrgyz Ministry of Health to strengthen regulatory review and approval processes, integrate quality assurance into procurement policies, and establish a system for monitoring the quality of TB medicines in the supply chain across the Kyrgyz Republic. In addition, we are supporting the development of guidelines to improve TB drug management and diagnostic testing algorithms, training on quality assurance for the National TB Program and the Ministry of Health, and development of other procedures and guidance documents to support TB drug management.
While much progress has been made in combatting DR-TB in the Kyrgyz Republic, much work is still left to do. Together, we are developing policies and procedures to improve quality management and working with key stakeholders to develop their capacity to guide sustained operations.
In the Kyrgyz Republic and across the globe, USP is helping countries build more resilient supply chains for medical products, strengthen national and regional regulatory authorities, expand regional manufacturing, and strengthen good storage and distribution practices to reduce medicine supply chain disruptions and build more resilient systems to ensure access to quality medical products for patients.
Now, more than ever, stronger medicine supply chains are essential to ensuring we preserve the hard-earned gains we’ve made against TB over the last decade.
We strive to build lasting relationships to produce better health outcomes for all.