Supporting Next Generation HIV-Prevention Options: Leveraging JSI’s experience in new product introduction
July 28th, 2021 | story
We know that rapidly identifying and scaling up HIV prevention, diagnostics, and treatment requires both supply and demand-side interventions. At JSI, we help shape and grow markets to maximize coverage of HIV (among other) products and services, and test and roll out new product introductions in more than 20 countries.
JSI strengthens health markets by connecting demand and supply-side initiatives to introduce and scale new products and technologies. We effectively facilitate market entry through our deep expertise in demand forecasting, market analysis, market shaping, data visualization, advocacy, and human-centered design. With our global expertise, we work with and coordinate with product developers across the value chain to identify critical gaps and market opportunities for new products that will help prevent the spread of HIV, reduce morbidity and mortality, and improve the quality of people’s lives.
We recognize that HIV programming requires new and innovative ideas which utilize evidence-based programming and consider absorptive capacity, and sustainability. Existing prevention methods have not done enough to stop the spread of HIV among key and priority populations, including adolescent girls and young women and gay men.
JSI is committed to supporting new HIV prevention options, including exciting new delivery mechanisms. Biomedical prevention is an active area of research and development of new ARV-based products such as long-acting injectable ARVs and implants, vaginal rings, and patches. A Cabotegravir long-acting injectable and the Dapivirine Vaginal Ring (DVR) are currently progressing through regulatory approval. Expansion of access to oral PrEP will provide an important platform for the introduction of new biomedical prevention interventions, allowing for the availability of new options for those who are not able to benefit from oral PrEP.
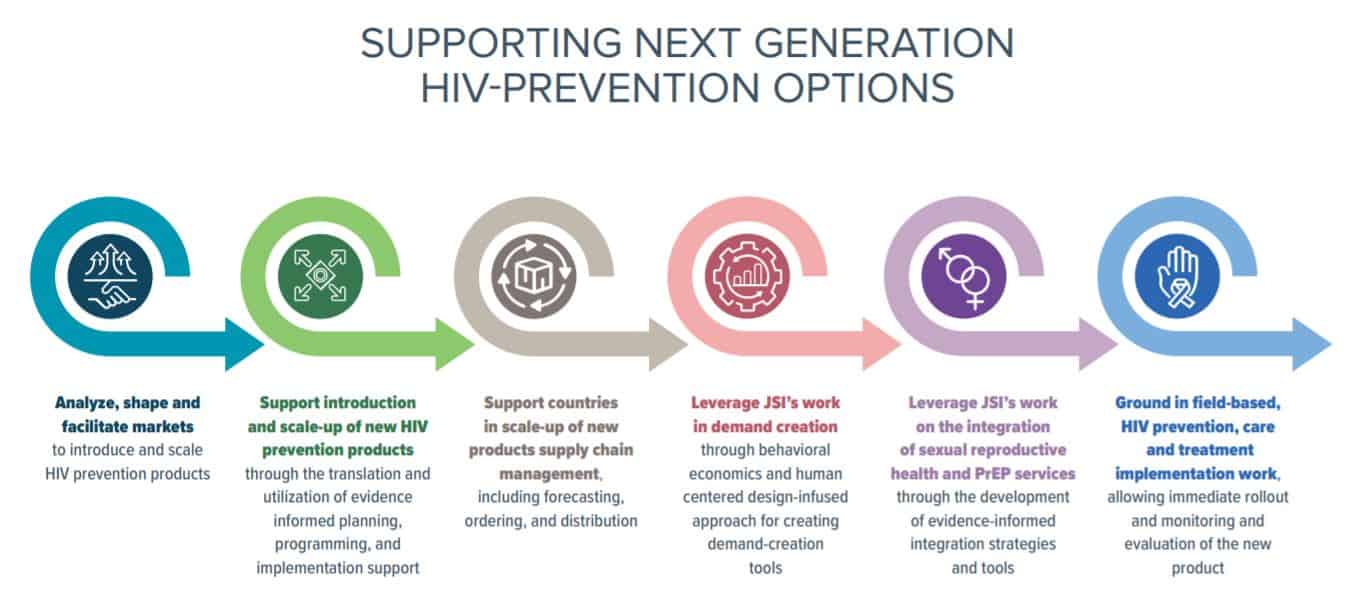
I recently met with three JSI colleagues to hear their thoughts on new HIV product introductions. Henry Nagai highlighted his experiences in Ghana on rolling out PrEP as a new intervention and his contributions to the Biomedical HIV Prevention Adaptable Product Introduction Framework guidance; Tanvi Pandit-Rajani talked about new product introduction, and specifically on market shaping and market analysis; and Johnnie Amenyah spoke about effective supply chain technical assistance for new products, as well as logistics and regulatory support. We frame these conversations in what we’ve done in the introduction of similar new products (PrEP, Sayana Press) and how we can apply these approaches to rolling-out new prevention methods. Tanvi, Johnnie, and Henry also discussed the various stages in supporting next-generation HIV prevention options:
We strive to build lasting relationships to produce better health outcomes for all.