Immunization Supply Chains: Creating a Better Design
July 27th, 2020 | viewpoint
Immunization supply chains (iSCs) are essential for ensuring access to vaccines that prevent diseases and protect the health of individuals, families, and communities. In every country, supply chains move vaccines from the port of entry through four or five different levels of storage until they reach health facilities, all while keeping them 2–8°C. Many countries struggle to manage changing characteristics of vaccines, new vaccines, and population growth because their iSCs were designed 40 years ago.
JSI is working with Ministries of Health and Expanded Program of Immunization stakeholders in four countries (Sierra Leone, Madagascar, Guinea, and Niger) that have committed to improving the iSC performance through a system design approach. A structured system design approach analyzes how different supply chain components interact and how changes in one or more components can improve vaccine availability and system efficiency, leading to higher immunization coverage and equity.
The approach begins with stakeholders agreeing on priorities and identifying potential scenarios for the iSC based on current supply chain bottlenecks. Collected data are used for computer modeling to analyze the scenarios, and JSI helps stakeholders agree on broad decision criteria including cost, equity, and feasibility of implementation.
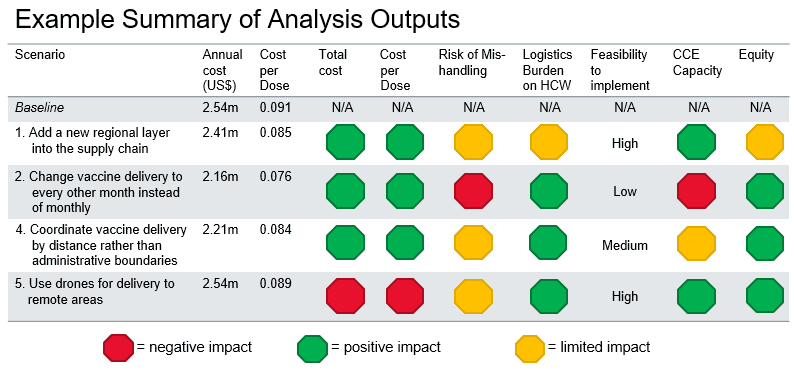
The results of this analysis have provided great insight into how the different iSCs could be changed to improve system efficiency and vaccine availability. Some changes, such as including oxytocin in the vaccine cold chain at the facility level, are minor and validate ad hoc approaches already in place. Other changes, such as adding regional-level cold stores, are much greater and would require more preparation, planning, and resources.
Insights include:
A system design analysis can help stakeholders identify potential efficiencies in the supply chain and plan for changes and required resources. Results of the analysis do not provide “the right answer,” but offer guidance that must be tailored to reflect country context and used for decision making. By creating supply chains that are more reliable and responsive, we can better prepare for systemic shocks caused by COVID-19 and other compromising events. This work was funded by Gavi, the Vaccine Alliance, and by UNICEF.
Written by: Wendy Prosser
We strive to build lasting relationships to produce better health outcomes for all.