A new COVID-19 vaccine will be a game-changer. Then what?
April 17th, 2020 | viewpoint
As the world’s pharmaceutical companies—and universities and research organizations—are all working to protect people against the COVID-19 pandemic, it is likely that there will be multiple COVID-19 vaccines that will reach domestic and international markets.
But the development of new COVID-19 vaccines is only the first step in protecting people. Supplying and delivering any new vaccine into a country—and to the population—involve complex processes.
Each vaccine will likely have a different profile and techniques for administration. There will be cold chain requirements and storage needs for each vaccine. Also, their deployment to the most at-risk populations will vary, and prioritization (as vaccine production and availability increase) will have to focus on which target populations receive the vaccine first.
Introducing new vaccines into established markets requires very specific, well-thought through plans. JSI has supported new vaccine introduction—from PCV to H1N1, HPV and rotavirus vaccines—in more than 30 countries. From what we have learned over many years, here are some of the critical things that must be considered by every country that will introduce a new COVID-19 vaccine:
Given the high demand and time that it takes to increase production, the initial COVID-19 vaccine supply will need to be prioritized and distributed to where the outbreaks are most acute. The vaccines will most likely not be available for everyone; therefore early vaccine use will be based on active surveillance and part of a broader prevention and control strategy (e.g., with testing, case tracking, etc). Focus in the early stages will be on those people most at-risk: health workers and health service providers, the elderly, those with immune-deficiencies, and those who care for and work with at-risk populations (e.g. in elderly care facilities and institutional environments where there are adults in close quarters).
Given that vaccines will be approved and available at different times (and potentially focused first on the domestic population in the manufacturer’s country), it is critical to develop strategies for who—and which countries—will receive vaccines when. Internationally, partners like WHO, UNICEF, and Gavi play a vital role in this.
Expedited registration for new products is needed. (For example, most countries developed these expedited procedures for H1N1 vaccine—they should be referenced and updated/adapted for COVID-19). Testing and regulation must remain rigorous while also being fast-tracked.
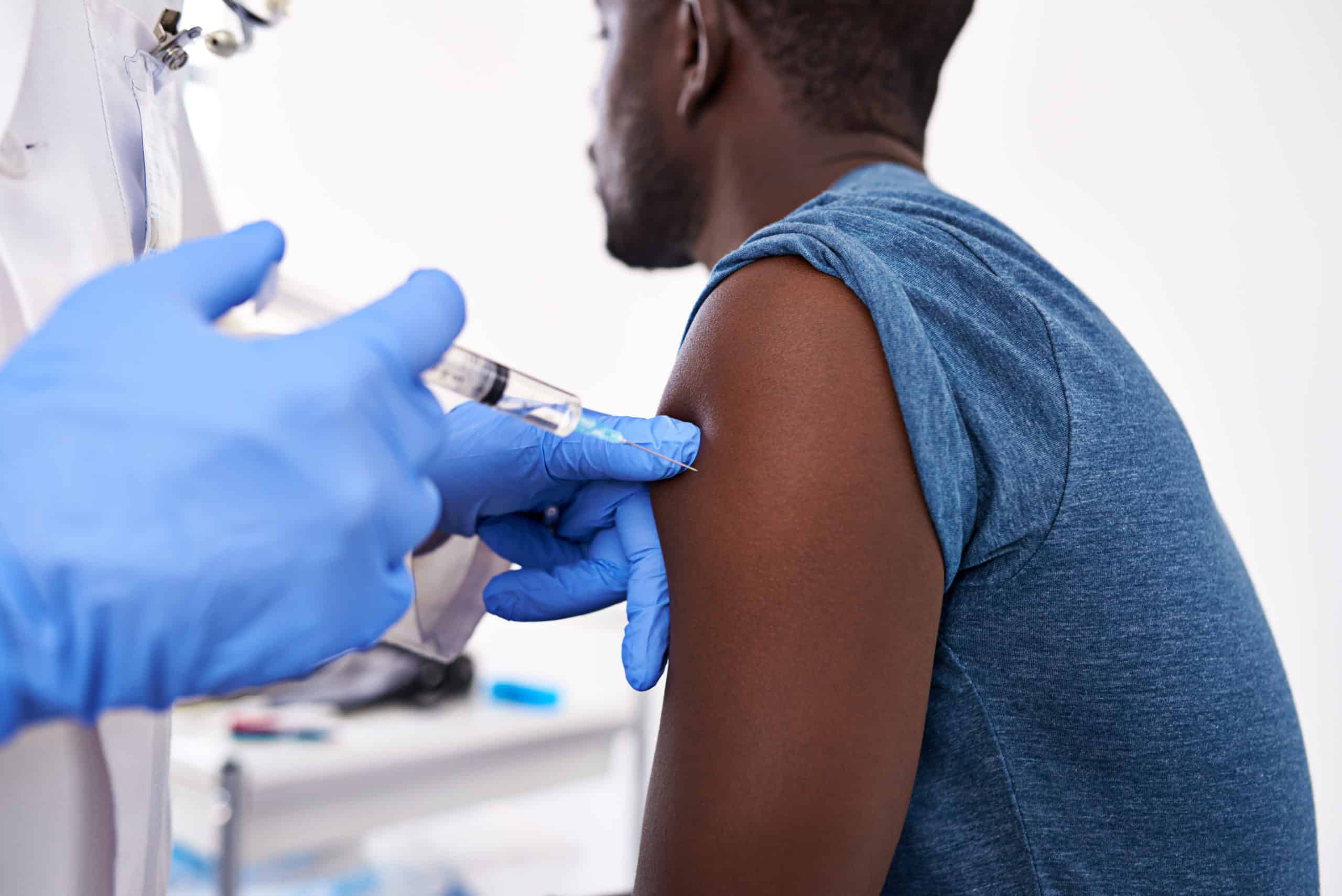
Because vaccines are made from living (or inactivated) organisms, each vaccine must be kept cold, typically between 2 and 8 degrees Celsius (or frozen, in the case of freeze-dried vaccines), throughout the supply chain: from manufacturer until it is provided to a client. Cold chains are very complicated!
When a vaccine is not kept at the specified temperature, it can lose its potency and effectiveness. This can be difficult in places with unreliable electricity—refrigerators may fluctuate on and off; and keeping temperatures stable during transport in a refrigerated truck or cooler is challenging, particularly traveling to a remote area in a country with weak infrastructure.
New technology is improving the performance of the vaccine cold chain, such as: solar refrigerators that are more reliable; remote temperature monitoring devices that alert a healthcare worker if a refrigerator goes out of the temperature range (so s/he can take quick action to resolve this), and better information technology to track vaccine stock levels at health facilities.
Given the different types of COVID-19 vaccines being developed, cold chain needs will vary. Most of the vaccines will likely require the normal temperature ranges of other vaccines and therefore fit within the current supply systems. Some, however, may take up more space, for example if they are provided in single dose, pre-filled syringes versus multi-dose vials. New technology is also being explored, such as the potential for micro-array patches where the vaccine may be stable at ambient temperature and can be administered via an adhesive patch placed temporarily on the skin. This technology will also need to pass through the clinical trial and approval processes.
Cancelled flights, closed ports, and furloughed or diverted workers are already causing challenges with vaccine supply and will impede the delivery of new vaccines. Health systems are being negatively impacted by COVID-19, with weaker systems suffering. Existing supply chain channels must be at-the-ready and strengthened to accommodate the new COVID-19 vaccines.
COVID-19 is an opportunity for governments to rethink their supply chains to create stronger, more nimble, and more resilient supply chains that can quickly adapt to emergencies such as the current pandemic.
Different products will require product-specific, easy-to-understand communications materials and technical guidance and support for health workers so they can correctly administer the vaccines, and store and handle them properly.
The general public also needs to be informed about when the vaccine is available, what protection the vaccine provides, and any potential side effects—as well as why some population groups may receive the vaccine before others.
So while the world anxiously awaits COVID-19 vaccines, now is the time to think through the steps needed for each country to prepare for those vaccine introductions. Vaccines will be a game-changer in the fight against coronavirus, but they have to be provided as part of a comprehensive strategy and through a health system that can support effective, equitable distribution.
By Lora Shimp and Wendy Prosser
We strive to build lasting relationships to produce better health outcomes for all.